Concrete
When the concrete is cured, the calcium silicate hydrate (C-S-H) is formed and the strength is developed. However, as byproducts, calcium hydroxide and ettringite are formed together to cause deterioration of concrete. Cured concrete is porous, so that the calcium hydroxide on the concrete surface easily reacts with moisture, CO2 gas, and acidic pollutants in the air to cause neutralization and corrosion of concrete, which may bring to serious structural defects.
In order to protect concrete from moisture, CO2 gas or acidic pollutants, the surface of concrete needs to be treated with ingredients, that can react with concrete and be integrated with the concrete. The treated concrete can be protected and its life can be extended.
Soluble Silicates
Soluble silicate which be dissolved in water is compound of silica (SiO2) and alkali metal (M2O). It is called soluble silicate or water glass. Sodium silicate, potassium silicate and lithium silicate are representative soluble silicates.
In order for silicate to be soluble, a certain proportion of alkali metal is absolutely needed relative to the silica content. The features of the soluble silicate are determined by the type of alkali metal, the molar ratio of silica to alkali metal and the concentration of the solution.
The difference of molar ratio and weight ratio of silica to alkali metal needs to be understood. For example, for a molar ratio of 3.2 of silica to alkali metal, the weight ratio in sodium silicate is 3.1, 2.0 in potassium silicate, and 6.4 in lithium silicate.
Figure 1 represents liquid silicates with the same molar ratio, considering molecular weight of each alkali metal. Even if silicates have the same molar ratio and concentration, the properties of each silicate may vary depending on the type of alkali metal.
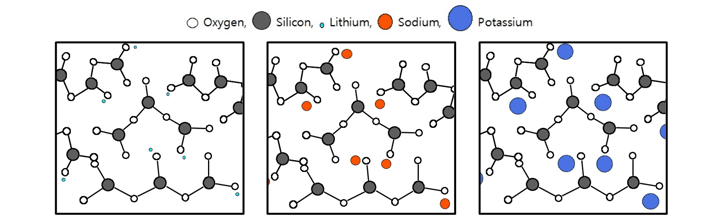
(a) Lithium Silicate (b) Sodium Silicate (c)Potassium Silicate
[Figure 1. Schematic representation of soluble silicates]
In liquid silicates, alkali metal ions, especially Na+, K+, Li+ ions, react very fast with acidic or ionic substances. For example, acids, water-soluble neutral salts, hydroxides, polyalcohol esters, etc. In addition, concentration change in solution, or even temperature change in solution brings to position change of alkali metal ions, which affects the properties of the liquid silicate. Position change of alkali metal ions means that silica molecular structures are also changed.
Reaction of Soluble Silicate and Concrete
Cured concrete is porous and there are relatively high excess calcium hydroxides in concrete surface layer. This is the result of excessive water rising up during curing. Calcium hydroxides on concrete surface easily react with moisture, CO2 gas, and acidic pollutants in the air and accelate deterioration of concrete. Soluble silicates are the best solution to this problem.
When soluble silicate designed to penetrate into concrete easily is applied on concrete surface, silicate penetrates into concrete pores and micro cracks, chemically reacts with calcium hydroxides and forms insoluble calcium silicate hydrates (C-S-H) in the concrete pore. Additional C-S-H becomes integrated with the concrete.
The oxygens at the end of the silica molecule in the soluble silicate exist in the state of ☰Si-OH and alkali metal ions near oxygens exist in the state of ☰Si-O-Na+, ☰Si-O-K+ or ☰Si-O-Li+. When the water evaporates or liquid silicate becomes unstable, polycondensation occurs between adjacent ☰Si-OH and ☰Si-OH, resulting in three-dimensional ☰Si-O-Si☰ amorphous network. When soluble silicate penetrates into concrete surface, monovalent alkali metal ions of silicate are substituted with Ca+2 ions of concrete and calcium silicate hydrates (☰Si-O-Ca-O-Si☰) are formed.
[Figure 2. Benefits of Soluble Silicates for Concrete]
Additional C-S-H in concrete pore increases density of concrete surface layer, so that the strength, abrasion resistance and absorption resistance of concrete are improved. Since the calcium hydroxides of concrete already reacted with silicate, concrete surface is stabilized chemically and does not react with moisture, CO2 and acidic pollutants in air. The durability of concrete treated with soluble silicate is improved and concrete life is extended.

[(a)Untreated concrete / (b)Treated concrete with silicate (additional C-S-H)]
[Figure 3. Treated concrete with soluble silicate (SEM)]
Bangsulee NS-100, Specialized Soluble Silicate for Concrete
Bangsulee NS-100 is a specialized soluble silicate designed for concrete waterproofing. Bangsulee NS-100 is a inorganic penetrating waterproofing agent for protection of concrete and extension of concrete life. Green Enhancement of Concrete!
All silicates are not excellent for concrete as waterproofing agents. General soluble silicate contains a large amount of alkali metal ions and it is viscous liquid. If they are applied to concrete as waterproofing agents, they cannot penetrate deep into concrete and cannot improve the density of concrete enough to prevent absorption of water. In addition, a lot of alkali metal ions occluded in silica network will be eluted by moisture or water, break down silica network and efflorescence occurs continuously. The key point in the technology of silicate based waterproofing agent is to implement durable bonds after reaction of silicate and concrete. Bangsulee NS-100 was developed based on the technology for specifically modified silicates of YOUNG IL CHEMICAL CO., LTD.
